[ad_1]
The aim of the current examine is to find out the required steps for dependable colocalization of NP inside lysosomes with a deal with CLSM. J774A.1 mouse macrophages had been used as a mannequin cell line for skilled phagocytes, since they’re able to phagocytosis (uptake of particles bigger than 1 µm) [42,43,44] however are additionally a widely known mannequin for endocytosis analysis [29, 45]. In that sense this is a perfect cell line to review a large spectrum of particle sorts fitted to the needs of this venture. Cells had been uncovered to amorphous silica NP as a result of they are often synthesized in several sizes and labelled with quite a lot of completely different fluorophores, permitting their monitoring in dwelling cells.
Fluorescent lysosomal probe traits
When designing an experiment, the important thing experimental parameters (i.e. LysoTracker probe choice and the soundness of fluorophores in addition to gear accessible) must be considered. Since lysosomes had been visualized by labeling the cells with LysoTracker probes, we first extracted the principle traits of LysoTracker probes (Desk 1). Experiments should be designed in a solution to reduce fluorescence cross-talk (i.e., overlap of fluorochromes’ excitation spectra) and bleed-through (i.e., overlap of fluorochromes’ emission spectra), both through the use of a free on-line spectral analyzer (accessible by Thermo Fisher Scientific) or experimentally (Further file 1: Fig. S1A) [46].
Affect of phenol crimson in cell tradition medium on fluorescence depth of LysoTracker probes
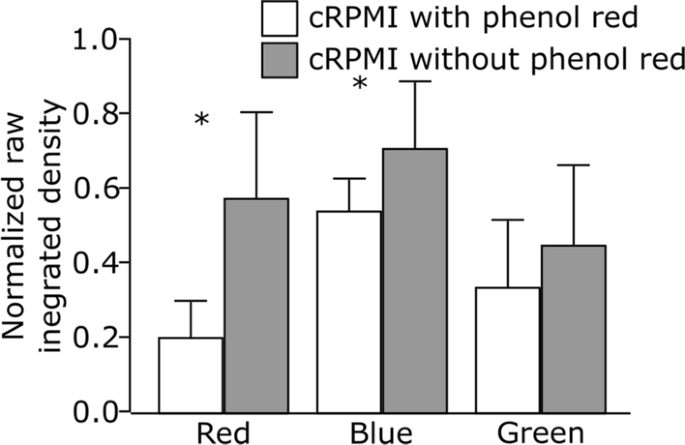
Our first try was to analyze whether or not the presence of phenol crimson, a routinely added pH indicator, within the tradition medium influences the fluorescence depth of the crimson, inexperienced and blue LysoTracker probes. Cells had been grown both in full Roswell Park Memorial Institute medium, supplemented with 10% fetal bovine serum (FBS), 1% (v/v) l-glutamine and 1% (v/v) penicillin/streptomycin (cRPMI) with or with out phenol crimson. After 24 h of incubation, the cells had been uncovered to LysoTracker probes and the impact was monitored below steady reside cell imaging. A consultant picture of a person cell for every LysoTracker probe could be present in Further file 1: Fig. S2. Our outcomes present that the presence of phenol crimson decreases the relative sign depth and biases the fluorescent sign as demonstrated by the numerous variations within the uncooked built-in density (i.e., sum of pixel values) of LysoTracker probes (Fig. 1). This distorts the correlation coefficients and the interpretation of the colocalization outcomes. The phenol crimson emits gentle within the blue and crimson area of the spectrum, inflicting primarily interference with the blue and crimson LysoTracker probes spectra (Further file 1: Fig. S1A). Alternatively, no important influence of phenol crimson was detected on the depth of LysoTracker Inexperienced. Primarily based on these observations, we propose utilizing phenol red-free cell tradition medium for experiments involving LysoTracker probes. In case using phenol red-containing medium can’t be averted, beneficial various is to make use of LysoTracker Inexperienced as a substitute.
Comparability of the normalized uncooked built-in densities of three completely different LysoTracker probes (crimson, blue and inexperienced) obtained by cell imaging in cRPMI with phenol crimson (white bars) and with out phenol crimson (grey bars). Error bars are normal deviation, n = 10 cells per remedy. *p < 0.05 by one-way ANOVA
Stability of LysoTracker Pink probe below steady and staggered imaging set-up
The power to hint the behaviour of lysosomes over longer durations opens a risk to review mobile processes upon extended and repeated publicity to NP. The suitability of LysoTracker probes for extended imaging was evaluated by monitoring the LysoTracker Pink fluorescent sign over a interval of 24 h in a steady experimental set-up. J774A.1 cells had been first stained with LysoTracker Pink for 1 h, the medium was changed with phenol red-free medium, and cells had been imaged reside for twenty-four h at 20 min intervals. Randomized datasets [17] had been recorded.
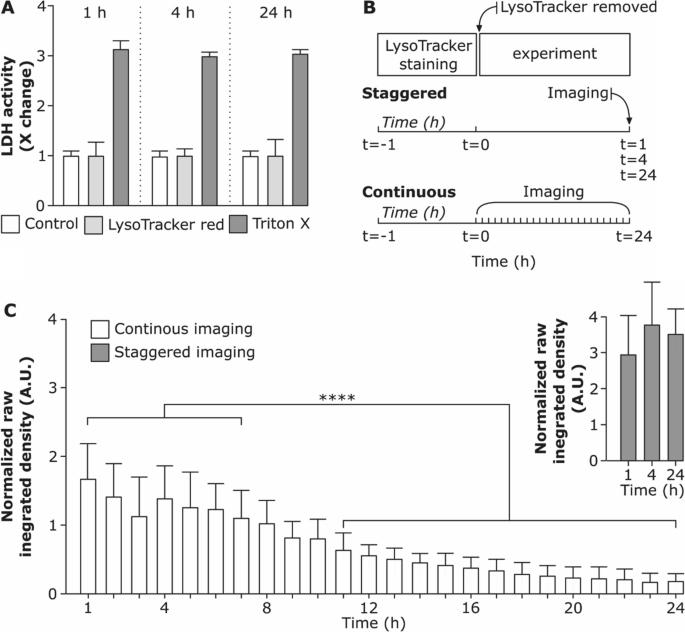
In a steady imaging set-up, the fluorescent sign of LysoTracker Pink didn’t considerably alter over the primary 8 h. Longer durations had been accompanied by a lower within the uncooked built-in depth of the LysoTracker Pink and elevated cell dying over time (Further file 1: Video S1). This remark is supported by Chen et al., who demonstrated that extended intracellular accumulation of LysoTracker probes causes a rise in intracellular pH and enhances quenching of the fluorescent dye [26]. Nonetheless, as assessed by lactate dehydrogenase (LDH) assay (Fig. 2A) we didn’t observe any important adjustments in LDH launch within the medium from LysoTracker handled cells in comparison with the management cells. Therefore, including LysoTracker probe doesn’t trigger cytotoxicity throughout the time vary investigated. Subsequently, we conclude that the cytotoxicity is reasonably influenced by phototoxicity, an impact brought on by in depth imaging.
Stability of LysoTracker Pink probe below completely different imaging situations. A Cell viability after incubation with LysoTracker Pink probe for 1 h, 4 h and 24 h assessed through membrane rupture assay (lactate dehydrogenase; LDH) and offered as fold change over untreated cells (management). Knowledge exhibits the imply of three replicates. Cells uncovered to 0.2% Triton X-100 (v/v) served as constructive management for LDH assay. B Schematic illustration of staggered experimental set-up (cells had been stained for 1 h with LysoTracker Pink probe, the supernatant containing LysoTracker Pink was eliminated and cells had been additional incubated and imaged at 1 h, 4 h and 24 h as hyperstacks) and steady set-up (cells had been stained for 1 h with LysoTracker Pink probe and imaged constantly for twenty-four h as hyperstacks). C Comparability of uncooked built-in densities of LysoTracker Pink probe versus time for steady and staggered imaging. Every time-point (hour) exhibits the uncooked built-in density measured for every cell individually. The whiskers are the usual deviations. **** (P < 0.05) is the numerous distinction analyzed by One-way ANOVA
To check whether or not there’s a distinction in LysoTracker Pink sign depth relying on the imaging situations an extra staggered experimental set-up was performed as proven in Fig. 2B. We noticed that the uncooked built-in density doesn’t differ considerably among the many staggered imaging set-up of 1 h, 4 h and 24 h after LysoTracker probe incubation (Fig. 2C). Nonetheless, a major lower in uncooked built-in density of LysoTracker Pink sign was present in steady live-cell imaging set-up and the impact collected with rising imaging time. This means that the fluorescent depth of the LysoTracker Pink probe drops principally due to the photobleaching impact as a result of steady imaging for a protracted time [54]. The impact may be cell-specific and is affected by the imaging settings.
Physicochemical traits of silica nanoparticles
As soon as the LysoTracker probe stability and imaging set-up are optimized, the choice of the optimum NP to evaluate the particular analysis query is essential. For our experiments, we chosen artificial amorphous silica NP of various sizes and labelled with completely different fluorophores.
Desk 2 represents the principle physicochemical traits of various sizes of silica (SiO2) NP (59 nm, 119 nm and 920 nm) used on this examine; NP of 59 nm and 119 nm dimension vary are internalized into cells primarily through pinocytosis whereas 920 nm SiO2 particles are taken up by phagocytosis [55]. A focus of 20 µg/mL of the NP suspension was chosen as this focus doesn’t impair cell viability [31] and all of the particles utilized in our research had been unfavourable for the endotoxin take a look at (information not proven). Comparability of the emission and absorption spectra of the completely different (nano)particles is proven in Further file 1: Figs. S1B and S1C.
The core particle diameter was measured by transmission electron microscopy (Further file 1: Fig. S3), whereas the hydrodynamic diameter was assessed each in Milli-Q water and full medium through dynamic gentle scattering (DLS). The corresponding histograms representing particle dimension distributions are proven in Further file 1: Fig. S4A and S4B. All SiO2 particles had been colloidally secure in Milli-Q and in cRPMI over 24 h of incubation time.
Confocal microscopy imaging and post-processing
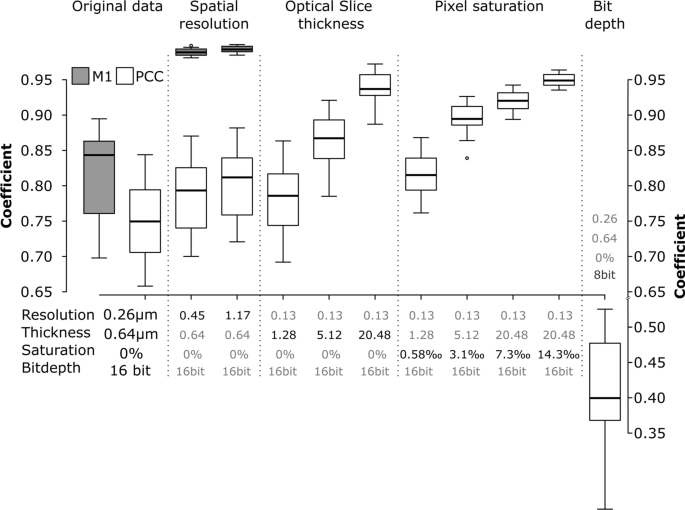
Even optimally designed experiments—no cross discuss, no bleed via—and pattern preparation could be undone by errors throughout pattern imaging [56]. Small variations within the settings can considerably alter the correlation coefficients [57]. Such results are proven in Fig. 3. Utilizing an optimally imaged, actual dataset, these biases had been demonstrated by simulating a set of various imaging situations for the next information elements: spatial decision, slice thickness, pixel saturation, and dynamic vary (Fig. 3).
Impact of spatial decision, optical slice thickness, pixel saturation and bit depth on the Pearson’s correlation coefficients (PCC, white packing containers) and Manders’ M1 coefficient (grey packing containers). The information correspond to the experimental set-up the place cells had been uncovered to 59 nm SiO2-BDP FL NP for 13 h, mounted and stained with Alexa Fluor 647-conjugated LAMP-2 antibody. The error bars signify the usual deviations. All the pictures had been analysed utilizing ImageJ with the JACoP plugin evaluating the correlation coefficients after every particular simulation
Decreasing the decision by lowering XY-pixel dimension (i.e., the gap between factors on a specimen, also referred to as “downsampling”) prompted a lower in Pearson’s correlation coefficient (PCC) and particularly in overlap (Manders, M1). Decrease resolutions trigger the combination of fluorescence indicators and thereby have an effect on the interactions between the 2 channels. That is most clear within the overlap (M1), proven within the gray boxplots of Fig. 3/Spatial decision. Subsequently, the optimum decision to picture the objects should be considered when organising the experiment.
Slice thickness of the optical slices additionally affected the colocalization parameters. Simulation of the opening of the pinhole—inflicting decrease axial decision, i.e. Z-axis thickness—yielded a rise in colocalization measures as objects alongside the Z-axis are more and more projected on prime of one another and find yourself falsely overlapping (Fig. 3/Optical slice thickness).
A brighter illumination will enhance the sign to noise ratio of the fluorescence sign, which can yield a decrease variance of the correlation coefficients. Nonetheless, the saturation of pixels will bias the consequence [58]. Saturation happens when the fluorescence depth exceeds the acquisition or storage restrict of the detector. Such clipping of knowledge has a major influence on correlation routines, similar to PCC, which attempt to clarify the depth of the primary channel with the data of the second. Our simulations present that even a fraction of saturated pixels as little as 0.3% of the overall pixel quantity will considerably alter the colocalization (Fig. 3/Pixel saturation). The pitfall of saturation could be utterly circumvented through the use of photon-counting detectors [59], which don’t convert the sign to binary output (see beneath) however retailer the precise variety of photons which have arrived on the detector. Nonetheless, such detectors are comparatively new within the discipline and never but widespread. Customary GaAsP-PMT detectors utilized in CLSM convert the analogue fluorescent intensities to a digital sign and retailer it on a pc. Often, we get the selection to save lots of the info with an data depth starting from 8 bit (28, i.e., 256 completely different depth values) as much as 16 bit (216, i.e., 65,536 completely different depth values). Recording information in 16 bit however saving the info at 8 bit, i.e., compressing the data, could trigger important shifts in PCC (Fig. 3/Bit depth) [60].
Earlier than continuing with additional experiments, all microscopy settings, similar to decision, optical slice thickness, saturation and bit depth must be considered for the reproducibility of the info.
Evaluation of nanoparticles throughout the lysosomes
On this part, we describe 4 completely different experiments illustrating the colocalization of silica NP with lysosomes and the corresponding interpretation. The PCC, M1 and M2 coefficients are expressed as a fraction/index of NP colocalized with lysosomes. The experimental design is matched to the particular intention of every examine.
Experiment I: the impact of steady reside cell imaging on colocalization
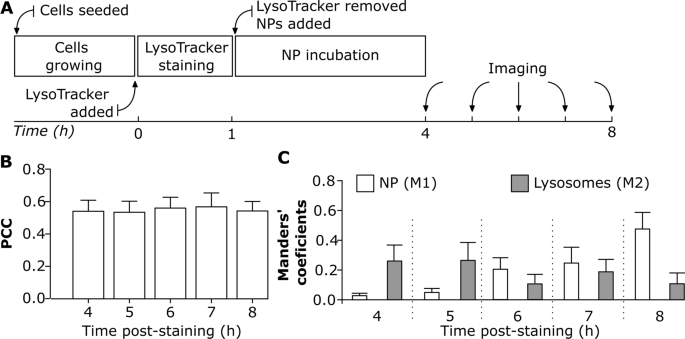
Understanding NP localization in lysosomes after their mobile uptake is essential for his or her use in biomedical functions [61]. The benefit of reside cell imaging is to get a greater perception into intracellular NP trafficking [19], and accumulation. The variability in NP uptake dynamics depends upon the cell kind [42] and cell division [19, 29, 62, 63]. The next experimental method enabled us to analyze whether or not NP colocalization with lysosomes varies over time below a steady imaging set-up. J774A.1 cells had been initially stained with LysoTracker Pink for 1 h after which sequentially uncovered to 59 nm SiO2-BDP FL NP for a length of 4 h to eight h (Fig. 4A). The colocalization was assessed utilizing PCC as an indicator of practical relationship and Manders’ coefficients (M1 and M2) as quantifiers of spatial overlap between fluorophores. PCC values point out that roughly 0.5 of intracellular NP fraction colocalized with lysosomes and there have been no important variations in PCC between 4 h and eight h (Fig. 4B). Nonetheless, the PCC doesn’t present detailed details about the distribution of NP arriving into lysosomes and the fraction of remaining empty lysosomes (Fig. 4B). In distinction, M1 and M2 coefficients differ between the completely different time factors (Fig. 4C) and supply data on the fraction of the NP co-occurring with lysosomes throughout the decision of a single voxel. We noticed that lower than 0.1 of NP fraction (M1) reached the lysosomes throughout the first 4 h of publicity (Fig. 4C). The fraction of NP that collected within the lysosomes elevated step by step over time as confirmed by rising M1 fraction as much as 0.5 after 8 h of incubation. Nonetheless, there was a variability within the fraction of lysosomes containing NP over time (M2). At 8 h time-point lower than 0.2 of the lysosomes fraction colocalized with NP, which is a decrease quantity in comparison with the 4 h time-point (Fig. 4C).
The impact of steady reside cell imaging on NP colocalization with lysosomes. A Experimental workflow: Cells had been first stained with LysoTracker Pink probe for 1 h, after which uncovered to twenty μg/mL of 59 nm SiO2-BDP FL NP in phenol free cRPMI. Cells had been imaged constantly from 4 to eight h. All the pictures had been analyzed utilizing ImageJ with a script (Script S2). Every cell was analyzed individually. B Pearson’s correlation coefficient (PCC) demonstrating correlation between NP and LysoTracker Pink probe (lysosomes) at completely different time factors. C Manders’ correlation coefficient M1 corresponds to the fraction of 59 nm SiO2-BDP FL NP inside lysosomes (stained with LysoTracker Pink) and M2 corresponds to the fraction of lysosomes crammed with NP. The corresponding histograms of fluorescence intensities of every channel are proven in Further file 1: Fig. S5A
Primarily based on completely different NP physicochemical properties similar to core materials, dimension, form, floor cost, porosity, floor roughness, floor space, crystalline construction, NP uptake and their intracellular destiny can differ over time. As a way to examine these phenomena, we monitored the adjustments in colocalization of NP of various sizes and integrated fluorophores.
Experiment II: the impact of various particles sizes on colocalization with lysosomes
A mixture of the PCC and Manders’ colocalization coefficients can present completely different messages of NP accumulation in lysosomes [39]. Since particles of various sizes are extensively used within the analysis, we selected 119 nm and 920 nm SiO2 particles labelled with the Cyanine5 (Cy5) fluorophore and examined their colocalization with lysosomes. Particles confirmed related zeta potentials (− 44 mV). After J774A.1 publicity to a single particle kind for twenty-four h, cells had been washed, stained with LysoTracker Pink probe for 1 h after which visualized by reside cell imaging (Fig. 5A).
Colocalization of different-sized SiO2 particles with lysosomes in J774A.1 macrophages. A Experimental workflow: Cells had been uncovered to SiO2 particles of various sizes for twenty-four h adopted by LysoTracker Pink staining for 1 h and imaged reside. B Consultant shiny discipline and confocal photographs of single cells with the corresponding cytofluorograms present colocalization of 119 nm SiO2-Cy5 particles (inexperienced) with lysosomes (crimson). C Colocalization of 920 nm SiO2-Cy5 particles (inexperienced) with lysosomes (crimson). Co-localized pixels seem in yellow. Scale bar: 10 µm. The corresponding histograms of fluorescence intensities of every channel is proven in Further file 1: Fig. S5A and the brilliant discipline photographs figuring out cell morphology in Further file 1: Determine S6. Quantification of colocalization of D. 119 nm SiO2-Cy5 particles and E 920 nm SiO2-Cy5 particles with LysoTracker Pink probe was carried out utilizing the ImageJ software program with JACoP plugin (n = 10 cells). Knowledge is offered as Pearson’s correlation coefficient (PCC) and Manders’ overlap coefficients, the place M1 represents the fraction of particles-associated pixels overlapping with lysosomes and M2 the fraction of lysosome-associated pixels overlapping with particles. Statistical evaluation was carried out utilizing unpaired t-test in GraphPad Prism software program: ***p < 0.001, ns: not important
Excessive accumulation of 119 nm SiO2-Cy5 particles inside lysosomes was noticed after 24 h of particle publicity as demonstrated by the PCC of 0.9. This means the particles uptake and intracellular sorting to the lysosomes. Such data can’t be extracted by the bare eye from the CLSM photographs however could be visualized by the cytofluorograms (Fig. 5B): the purpose cloud expanded in the direction of the highest proper nook, and the rise in PCC mirrored this course of. As represented by Manders’ coefficients, virtually all internalized 119 nm SiO2-Cy5 particles had been related to the lysosomes (M1 > 0.9) after 24 h. As well as, there have been nonetheless lysosomes accessible with out particles, as demonstrated by 0.3 of lysosome fraction (M2, Fig. 5D).
A distinct final result was noticed with the 920 nm SiO2-Cy5 particles (Fig. 5C, E). Solely 0.3 of the particles fraction arrived to the lysosomes (PCC: 0.3) at 24 h upon particle publicity. Manders’ coefficients present that solely 0.4 of particles fraction reached lysosomes inside 24 h (M1: 0.4) and the vast majority of lysosomes fraction (M2: 0.2) had been nonetheless left with out particles (M2, Fig. 5E). Thus, micron-sized particles had been much less more likely to enter the lysosomes inside 24 h and presumably sedimented within the cells’ environment or collected in different intracellular compartments [64,65,66]. A doable clarification for decrease colocalization of larger particles with lysosomes might be as nicely the variations in particle quantity added to the cells (9 × 109 of 119 nm SiO2-Cy5 particles per mL vs 2 × 107 of 920 nm SiO2-Cy5 particles per mL) or, alternatively, completely different mobility of particles throughout the cells [67].
Experiment III: colocalization between NP with completely different fluorophores and lysosomes
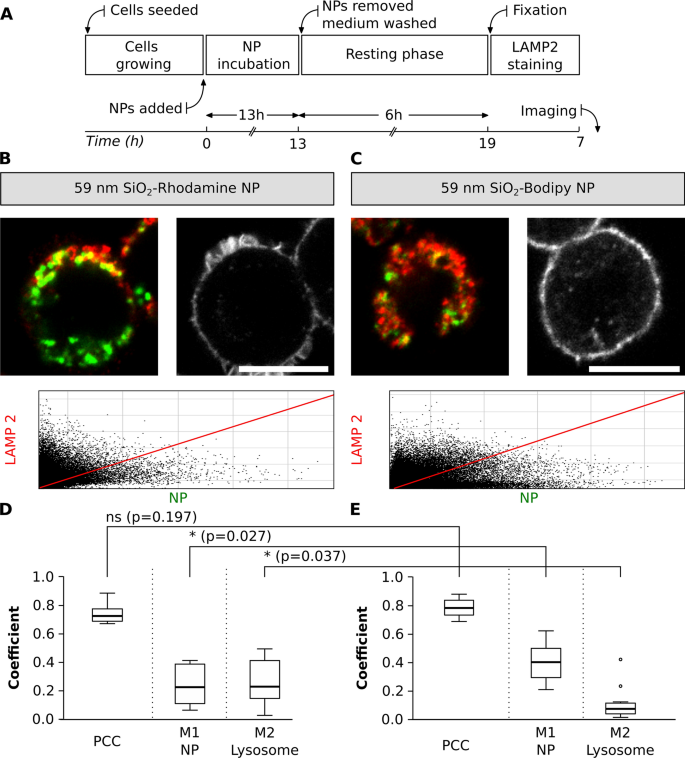
A possible colocalization pitfall is the dependence of fluorescent dye encapsulation on the physicochemical properties of NP. The dye encapsulation would possibly change the physicochemical properties of NP and the fluorescence properties of a dye, for instance, by inducing fluorescence quenching or enhancing emission [25, 68, 69]. An experimental design was carried out to check the colocalization of NP with related sizes however completely different fluorophores. J774.A1 cells had been initially uncovered to 59 nm SiO2-BDP FL NP and 59 nm SiO2-Rhodamine B (RhoB) NP for a interval of 13 h. The medium containing NP was subsequently eliminated and cells had been washed. Subsequent, a contemporary medium with out NP was added and a resting section of 6 h was carried out to analyze whether or not all of the NP which have simply reached the cell membrane continued being internalized and accumulate within the lysosomes. The resting time was chosen based mostly on identified literature, because it has been proven that completely different NP or macromolecules attain the lysosomes in a interval from 3 to six h [19, 70,71,72,73,74]. Lastly, the cells had been mounted and stained with LAMP-2 conjugated with a secondary antibody Alexa Fluor 647, which was chosen as a result of doable mixture with NP-conjugated fluorophores (Fig. 6A). The cells uncovered to 59 nm SiO2-BDP FL NP and 59 nm SiO2-RhoB NP had been stained with LAMP-2 and imaged (Fig. 6B and C).
Colocalization between NP with completely different fluorophores and lysosomes. A Experimental workflow. Consultant photographs of single cells uncovered to 2 completely different SiO2 NP (inexperienced), B SiO2-RhoB NP and C SiO2-BDP FL NP. Lysosomes had been stained with LAMP-2 antibody (proven in crimson). Scale bar: 10 µm. The corresponding histograms of fluorescence intensities of every channel are proven in Further file 1: Fig. S5B and the F-actin staining figuring out cell morphology in Further file 1: Fig. S6. The plots signify primary parameters (PCC, M1 and M2) to estimate colocalization between completely different NP and lysosomes. The colocalization between the D 59 nm SiO2-RhoB NP and E 59 nm SiO2-BDP FL NP with lysosomes, labelled with LAMP-2 antibody. PCC: Pearson correlation coefficient, M1 and M2: Manders’ correlation coefficients. The whiskers signify the usual deviations. Evaluation was carried out in particular person cells for every experiment (n = 10–13 cells). All the pictures had been analysed utilizing ImageJ, with the JACoP plugin evaluating the correlation coefficients. Statistical evaluation was carried out utilizing unpaired t-test in GraphPad Prism software program. * p < 0.05, ns: not important
Primarily based on PCC values, the outcomes confirmed no important variations within the colocalization of lysosomes and NP with completely different fluorophores (PCC: 0.8). Nonetheless, Manders’ correlation coefficients (M1, fraction of NP contained in the lysosomes) confirmed that 0.2 of SiO2-RhoB NP fraction are localized within the lysosomes (Fig. 6D) in comparison with 0.4 of SiO2-BDP FL NP fraction (Fig. 6E). Moreover, a special fraction of lysosomes crammed with NP (M2) was noticed when evaluating NP with completely different fluorophores, 0.3 for the cells uncovered to SiO2-RhoB NP and 0.1 for the cells uncovered to SiO2-BDP FL NP. This might be defined by the distinction in zeta potential between SiO2-BDP FL NP (− 52 mV) and SiO2-RhoB NP (− 34 mV). As reported beforehand in literature, NP uptake and their intracellular destiny are strongly influenced by the NP floor properties [6, 69, 75,76,77].
Experiment IV: comparability of NP colocalization with lysosomes between fixed-and live-cell imaging
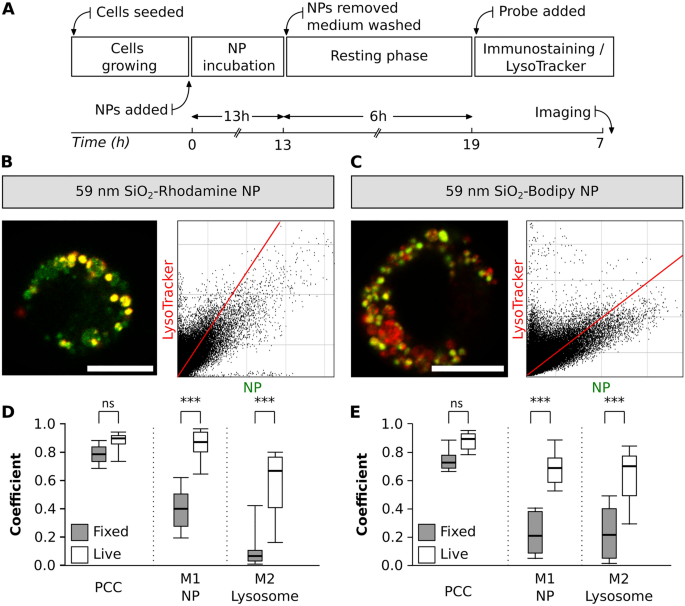
When performing lysosomal staining, each LysoTracker probes and LAMP antibodies can be found. Nonetheless, there are variations each of their specificity in addition to in staining protocols, which may considerably influence the experimental final result [27]. With this experimental method, the adjustments in colocalization of NP with lysosomes between live- and fixed-cell imaging had been evaluated. The identical publicity time and resting section was stored as described in part III (Fig. 7A). Along with the LysoTracker staining, cells had been immunostained with LAMP-2 antibody (Fig. 7B and C) and the outcomes from fixed-cells had been in comparison with the info collected in part III.
NP colocalization with lysosomes between fixed-and live-cell imaging. A Experimental workflow. Consultant photographs of a single cell uncovered to 2 completely different NP sorts, B SiO2-RhoB NP (inexperienced) and C SiO2-BDP FL NP (inexperienced). Lysosomes had been stained with LAMP-2 antibody (crimson). The corresponding histograms of fluorescence intensities of every channel are proven in Further file 1: Fig. S5B. The graphs depict the principle parameters (Pearson’s correlation coefficient—PCC, Manders’ correlation coefficients—M1 and M2) to estimate the colocalization between D SiO2-RhoB NP and lysosomes (LysoTracker Inexperienced) and E SiO2-BDP FL NP and lysosomes (LysoTracker Pink) in reside cells (white field) and glued cells stained with LAMP-2 (gray field). The whiskers are the usual deviations. The evaluation was completed in particular person cells for every experiment (n = 8–13 cells). All the pictures had been analysed utilizing ImageJ with the JACoP plugin evaluating the correlation coefficients. Statistical evaluation was carried out utilizing unpaired t-test in GraphPad Prism software program. ***p < 0.001, ns: not important
No important adjustments in PCC values had been noticed amongst reside and glued cells for each NP sorts. Nonetheless, we noticed variations for Manders’ correlation coefficients (M1 and M2) (Fig. 7D and E). In reside cells, uncovered to 59 nm SiO2-RhoB NP, there have been roughly 0.9 of NP fraction colocalized with the lysosomes (M1), however in fixed-cell the colocalization decreased to 0.4 (Fig. 7D). This distinction is as nicely evident within the cells uncovered to 59 nm SiO2-BDP FL NP the place 0.7 of the NP are colocalized with the lysosomes of dwelling cells stained with LysoTracker in distinction to solely 0.2 of colocalized NP contained in the lysosomes of fixed-cells (Fig. 7E). Likewise, a lower within the fraction of lysosomes crammed with NP (M2) between reside and glued cells was famous. These variations are attributed to picture processing, and selectivity of LysoTracker probe (stains lysosomes and late endosomes) vs LAMP-2 (particular to LAMP-2 protein current on lysosomal membrane) [78].
Correlative research between reside and glued cells are difficult as a result of the form of the mounted cells varies from its pure state [79]. Furthermore, a quantitative evaluation that compares and correlates the intensities of fluorophores such because the LysoTracker probes after cell fixation is anticipated to be much less exact as a result of lower of LysoTracker depth and additional adjustments in mobile form (Further file 1: Figs. S7 and S8). We have now already described that every experimental issue that alters the depth of the fluorophores could have repercussions, particularly on the Manders’ coefficients, as proven in Further file 1: Figs. S8 and S9. In conclusion, many parameters affect the NP-lysosome colocalization final result such because the fluorophore dye to label the NP, the staining process for lysosomes in cells, the lysosome marker specificity, and the imaging set-up. Subsequently, it is suggested to contemplate these parameters when planning and conducting the experiments in addition to to regulate every experiment individually based mostly on the analysis query to attenuate the variability.
[ad_2]